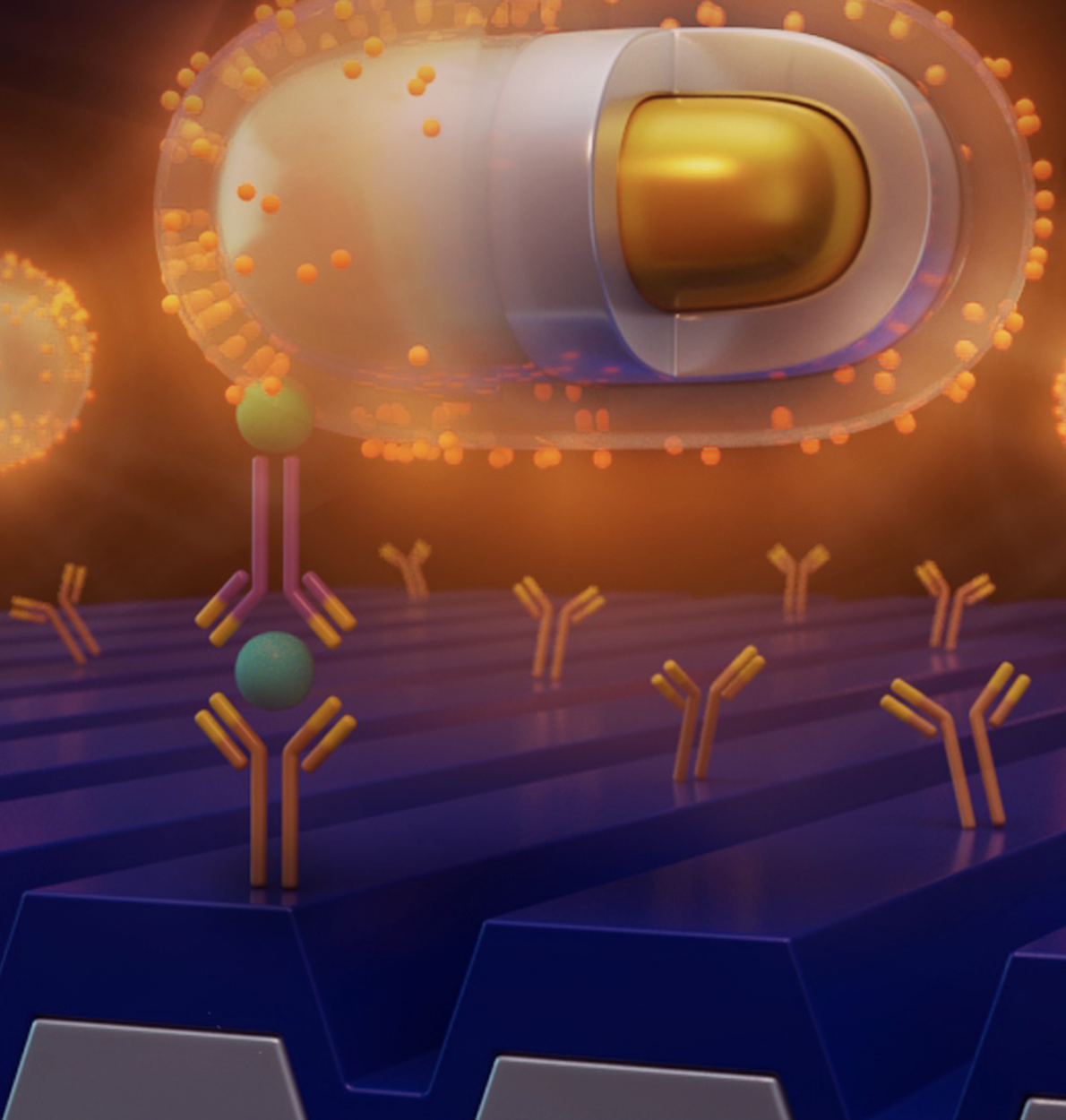
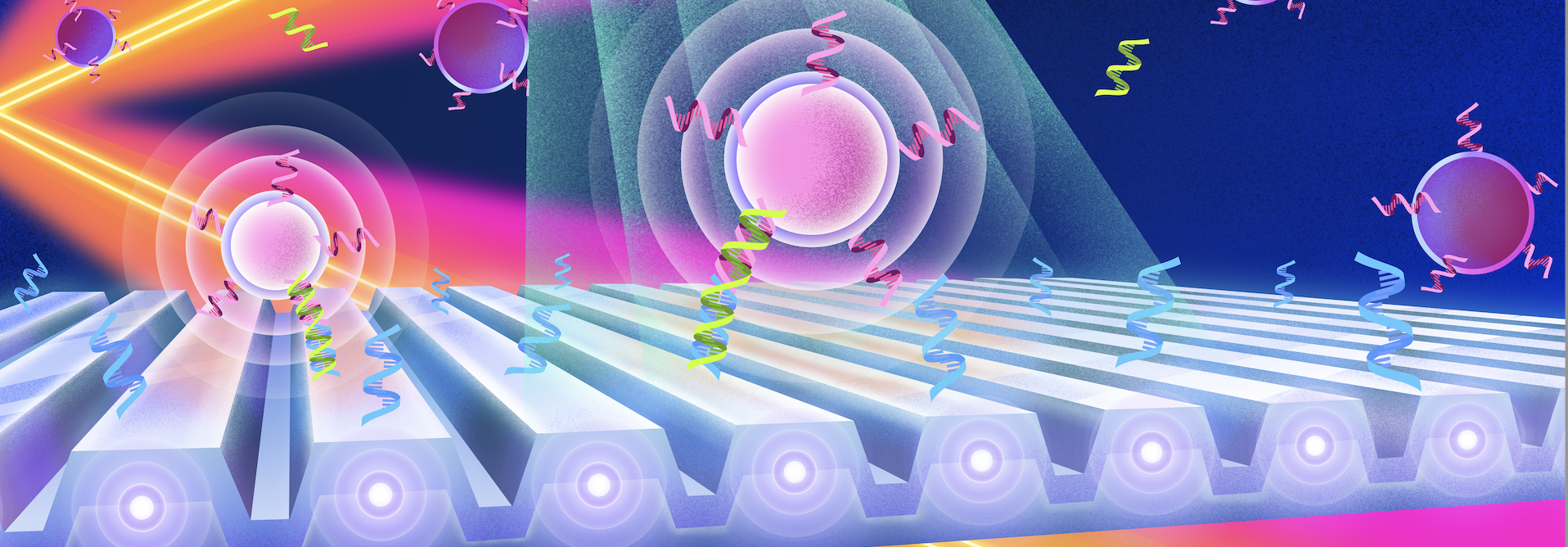
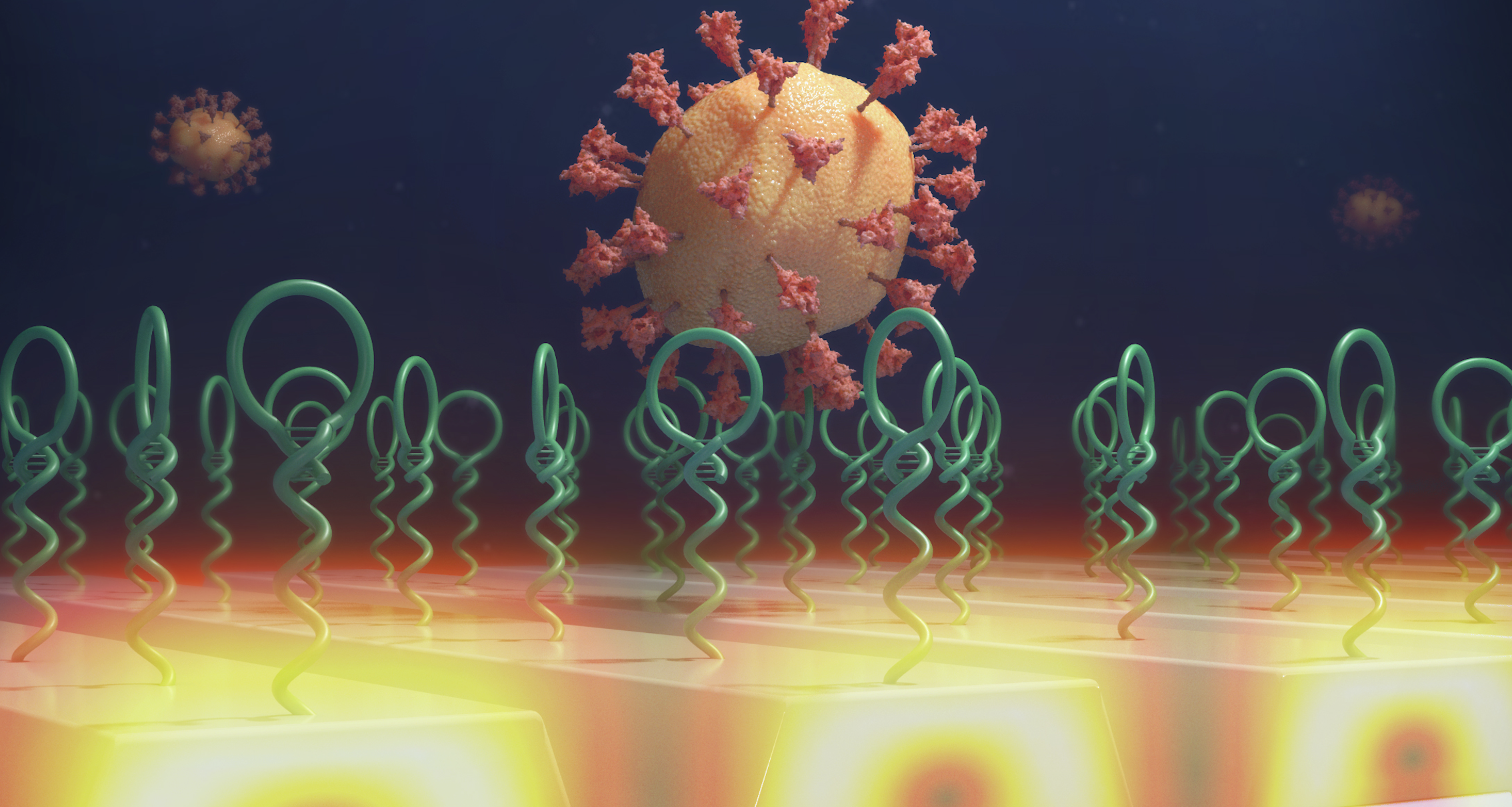
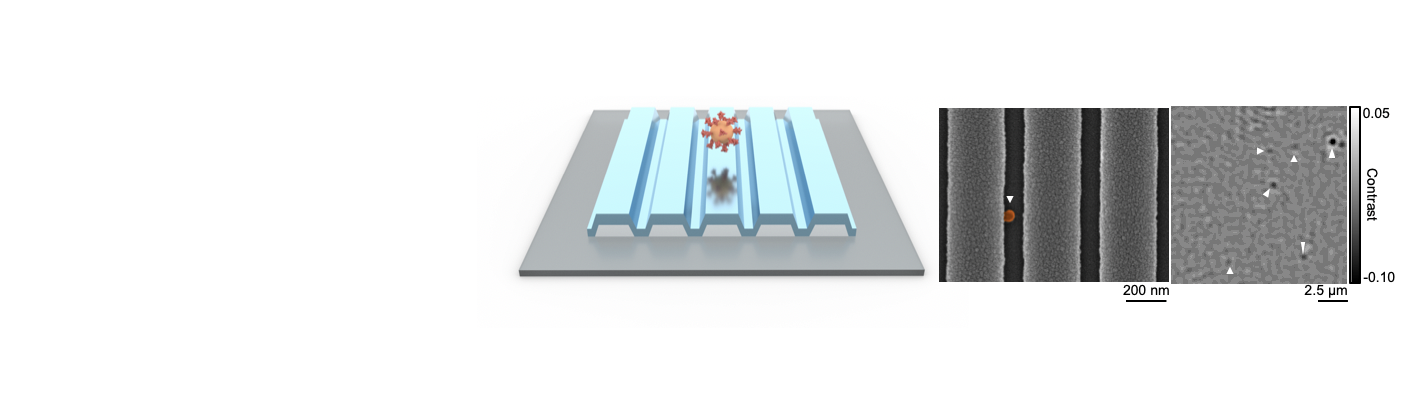
Researchers from the Cancer Center at Illinois (CCIL) Program Leader Brian Cunningham’s lab in collaboration with researchers at Washington University have demonstrated a new capability to detect and count individual biomolecules at low concentrations. This technology may significantly improve the efficacy of current cancer detection and measurement methods. Biomarkers play a significant role in diagnostics because their […]